What is an MDSAP Audit Checklist?
An MDSAP audit checklist is a tool used by quality managers to determine if the manufacturer’s QMS meets the requirements of ISO 13485:2016 and that of Regulatory Authorities participating in the MDSAP. MDSAP audit checklists also help quality managers determine the readiness of medical device manufacturers for MDSAP certification by recognized third-party Auditing Organizations (AO).
What is MDSAP and Why is it Important?
The MDSAP is a certification program for the QMS of manufacturers that wish to sell medical devices to the following MDSAP participating countries: the USA, Australia, Japan, Brazil, and Canada. With that, the Regulatory Authorities (RAs) participating in this program are the following:
- Australian Therapeutic Goods Administration (TGA)
- Brazil’s Agência Nacional de Vigilância Sanitária (ANVISA)
- Health Canada
- Ministry of Health, Labour and Welfare (MHLW)/Pharmaceuticals and Medical Devices Agency (PMDA) (Japan)
- US FDA
Initiated by the International Medical Device Regulators Forum (IMDRF), MDSAP intends to create a consolidated process for maintaining the safety and quality of medical devices by manufacturers that meet QMS international standards (like ISO 9001 and ISO 13485:2016) and comply with the requirements of Regulatory Authorities (RA) of participating countries.
How to Prepare for MDSAP Certification
To guide you as you prepare for acquiring this certification, here are some steps and tips you can follow:
- Gain an understanding of the ISO 13485:2016 standard and the RA requirements of the country where you are to sell medical devices.
- Identify areas for improvement in the current QMS by performing a gap analysis or a readiness audit to ensure adherence to ISO 13485:2016 and other requirements.
- Conduct quality monitoring audits and maintain a record of results.
- Define your organization’s competencies and determine training requirements for MDSAP certification based on the audit results.
- Ensure competency needs are met and that all parties involved are kept in the loop.
- Partner with an Auditing Organization for MDSAP certification.
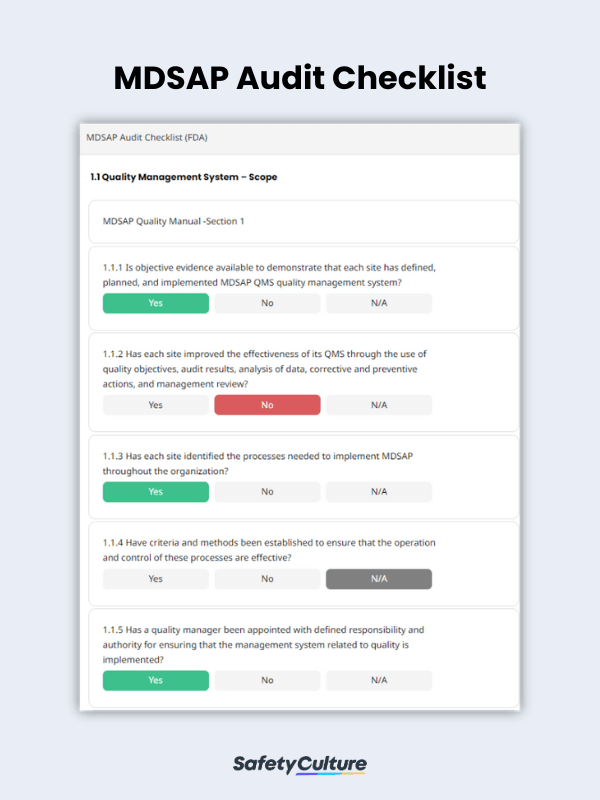
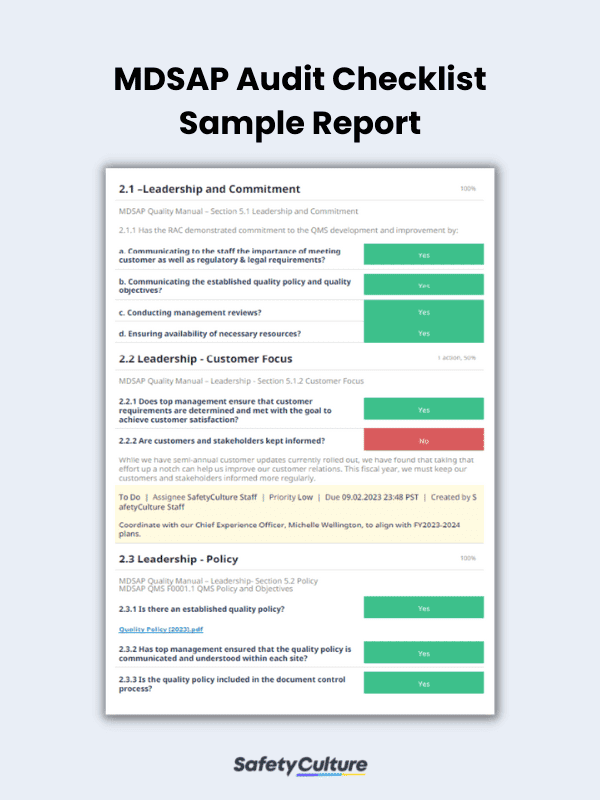
What to Include in an MDSAP Audit Checklist
To help you conduct an in-depth QMS audit according to MDSAP requirements, use the following guide on what you must put in your MDSAP audit checklist:
- Title Page – includes the manufacturing site name, date and location of the audit, and name of the auditor
- Individual Sections for Each QMS Requirement – allows you to verify if the manufacturing site follows the standards stated in the MDSAP quality manual
- Completion Page – where you can provide comments and recommendations, if any, and sign off the report by including the name and signature of the auditor
FAQs About MDSAP Audits
Currently, the MDSAP certification is voluntary in Australia, Japan, Brazil, and the United States. However, the program is mandatory for medical device manufacturers in Canada. Since the MDSAP only has these 5 participating countries, its requirements aren’t necessarily adopted by other nations and regions.
An average of 15 minutes is usually spent on the actual auditing process in a medical device manufacturing company to check its compliance with MDSAP. However, the entire process of an MDSAP audit consists of a series of 3 audits throughout a 3-year period:
- Initial certification audit
- Surveillance audit
- Recertification audit
In most cases, being ISO-certified is a prerequisite for acquiring an MDSAP certification. However, ISO and MDSAP are two different programs that follow similar requirements. While some countries can be compliant with ISO standards, only the 5 participating countries recognize the MDSAP certification.